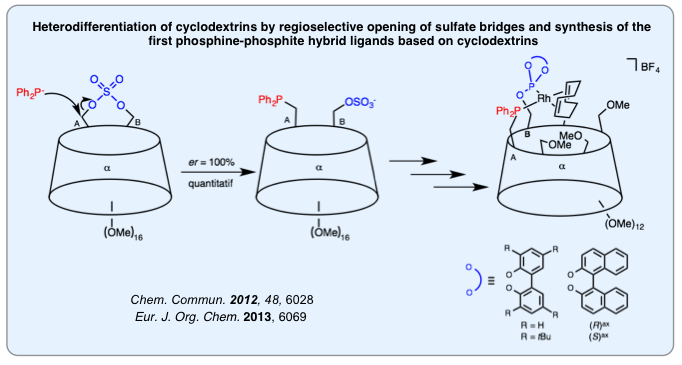
Regioselective Functionalization of Cyclodextrins
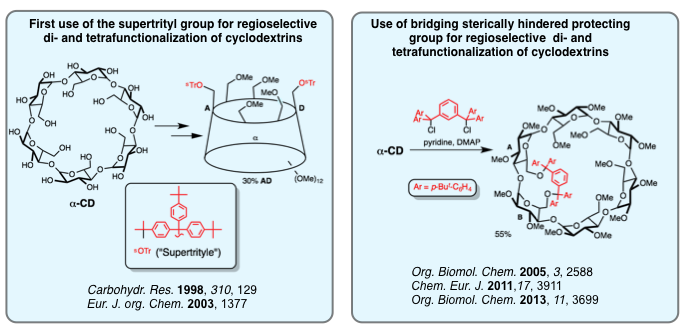
Straightforward synthesis of gram-scale quantities of cyclodextrin derivatives bearing various functional groups at specific locations is key to accessing cyclodextrin-based polydentate ligands. Over the years, we have developed a general strategy relying on sterically hindered trityl derivatives that allowed us to prepare a number of di- and tetrafunctionalized methylated cyclodextrins. We have also found a new way for heterodifferentiating cyclodextrins by opening cyclodexrin-capped sulfates with sterically hindered nucleophiles, in particular phosphides, so as to access hybrid P(III) ligands. Ongoing research is aimed at extending the variety of functional groups that can be placed at specific locations on the macrocyclic platforms.