Cyclodextrin-based P(III) Ligands
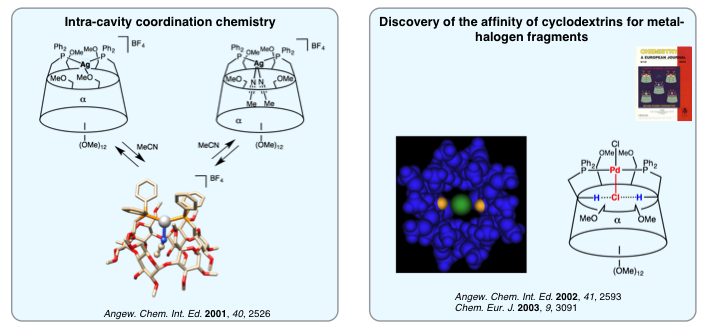
We were among the first groups to associate the ubiquitous P(III) donor atom with cyclodextrins. This was achieved by reacting a methylated cyclodextrin platform having leaving groups at specific locations on the narrow rim of the cyclodextrin (primary face) with various phosphides. Whether monodendate or polydendate, the resulting ligands are all capable of keeping one or several metal centers rigidly positioned close to the cavity. Such a feature was made possible by introducing a coordinating handle onto the cyclodextrin platform or by means of large chelate complexes. During the course of our studies, we managed to develop an original intracavity coordination chemistry allowing us to control the metal first coordination sphere. In this respect, marked affinity of the cyclodextrin cavity for chlorido ligands was revealed in chelate complexes displaying M-Cl bonds.
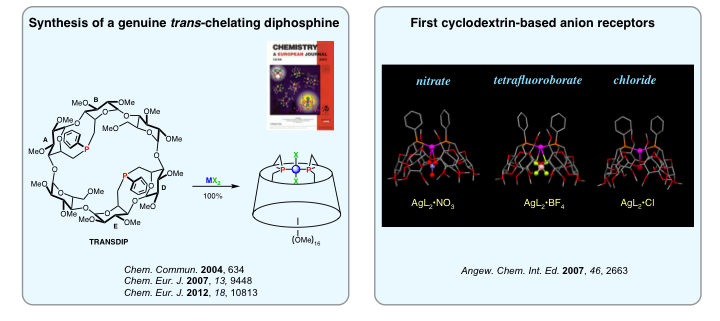
In 2004, we designed the first diphosphine behaving exclusively as a trans-chelating ligand. This is of great importance in coordination chemistry as the quest of such ligands has remained a challenge for more than 100 years. This diphosphine, christened TRANSDIP, is capable of stabilizing unprecedented organopalladated species thanks to its confining properties. As for its silver complexes, they behave as ditopic receptors towards middle sized anions.
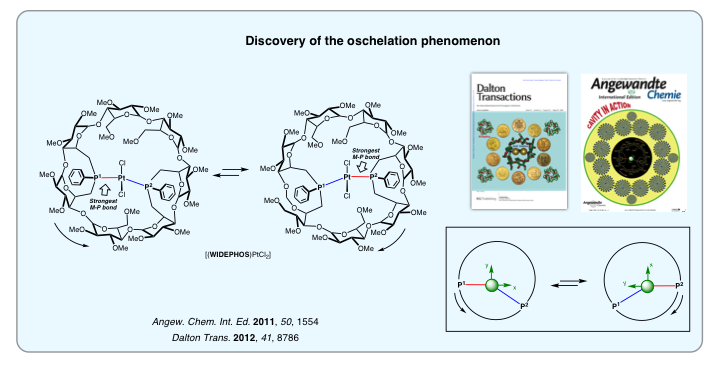
In 2011, we achieved the synthesis of the β-cyclodextrin analog of TRANSDIP (WIDEPHOS). Because of the large distance between the two non equivalent phosphorus atoms and the great rigidity of the bidendate ligand, WIDEPHOS gave rise to trans-chelate complexes in which the donor atoms are bonded to the metal center with unequal strength. This non optimal chelation is responsible for an unprecedented molecular motion christened “oschelation”. WIDEPHOS is also perfect for synthesizing dinuclear complexes with unusual coordination spheres.
In 2011, we achieved the synthesis of the β-cyclodextrin analog of TRANSDIP (WIDEPHOS). Because of the large distance between the two non equivalent phosphorus atoms and the great rigidity of the bidendate ligand, WIDEPHOS gave rise to trans-chelate complexes in which the donor atoms are bonded to the metal center with unequal strength. This non optimal chelation is responsible for an unprecedented molecular motion christened “oschelation”. WIDEPHOS is also perfect for synthesizing dinuclear complexes with unusual coordination spheres.
Cyclodextrin-based Thioethers
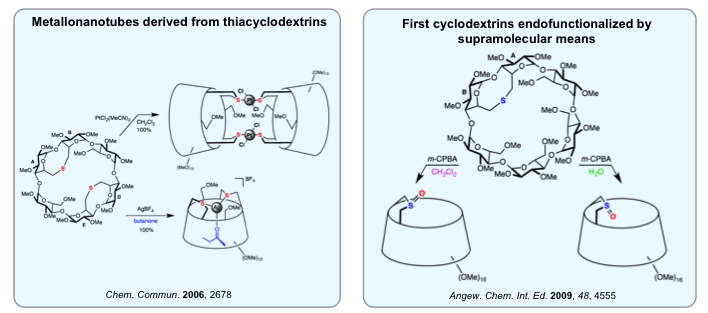
Thioether analogs of cyclodextrin-based P(III) ligands have also been synthesized. They form either intracavity complexes or very rigid metallonanotubes depending on which lone pairs is involved in the complexation process. Some of these thioethers can also be oxidized in a diastereoselective manner. The observed diastereoselectivity proved to be highly solvent dependent.
Current research is aimed at extending the synthetic methology we developed for P(III) and sulfur ligands to other donor atoms such as nitrogen so as to diversify our portfolio of confining ligands.