Intracavity Transition Metal Catalysis
By forcing the cyclodextrin cavity to act as a second sphere of coordination, we were able to alter the catalytic properties of several encapsulated transition metal cations both in terms of activity and selectivity. So far, strong emphasis has been placed on carbon-carbon bond forming reactions, such as the Heck and Suzuki couplings as well as hydroformylation and olefin oligomerizations/polymerizations. Because cyclodextrins are optically active host molecules, they also very attractive for asymmetric catalysis. High optical inductions have been achieved in ubiquitous asymmetric hydrogenation and hydroformylation reactions as well as in the less common asymmetric polymerization of pro-chiral olefins.
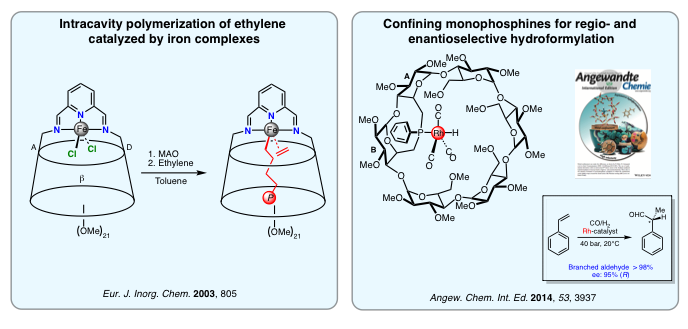
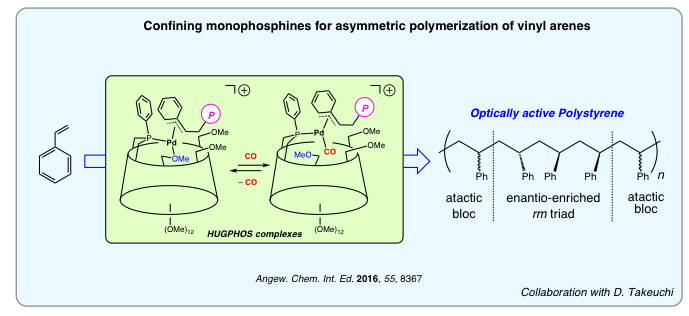
We are currently looking at increasing the scope of our confining ligands by testing other transition metal-catalyzed reactions, including asymmetric ones. Particular attention will be paid to reactions taking place in water to deal with environmental issues such as recyclability and the use of non toxic solvents.