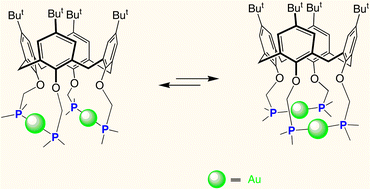
19.
Playing with podands based on cone-shaped cavities. How can a cavity influence the properties of an appended metal centre?
C. Jeunesse, D Armspach, D. Matt, Chem. Commun. 2005, 5603-5614.
Playing with podands based on cone-shaped cavities. How can a cavity influence the properties of an appended metal centre?
C. Jeunesse, D Armspach, D. Matt, Chem. Commun. 2005, 5603-5614.
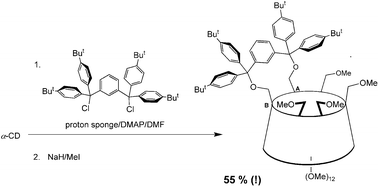
18.
A new approach to A,B-difunctionalisation of cyclodextrins using bulky 1,33bis[bis(aryl)chloromethyl]benzenes as capping reagents
D. Armspach, L. Poorters, D. Matt, B. Benmerad, F. Balegroune, L. Toupet, Org. Biomol. Chem. 2005, 3, 2588-2592.
A new approach to A,B-difunctionalisation of cyclodextrins using bulky 1,33bis[bis(aryl)chloromethyl]benzenes as capping reagents
D. Armspach, L. Poorters, D. Matt, B. Benmerad, F. Balegroune, L. Toupet, Org. Biomol. Chem. 2005, 3, 2588-2592.
17.
Effective A,B-disubstitution of cyclodextrins using capping reagents containing two”trityl”subunits
L. Poorters, D. Armspach, D. Matt, B. Benmerad, F. Balegroune, Proceedings of the 12th International Cyclodextrin Symposium, Montpellier, France, APGI, 2004, 117-120.
Effective A,B-disubstitution of cyclodextrins using capping reagents containing two”trityl”subunits
L. Poorters, D. Armspach, D. Matt, B. Benmerad, F. Balegroune, Proceedings of the 12th International Cyclodextrin Symposium, Montpellier, France, APGI, 2004, 117-120.
16.
Phosphinidene-capped cyclodextrins – A new generation of introverted ligands.
L. Poorters, D. Armspach, E. Engeldinger, D. Matt, Proceedings of the 12th International Cyclodextrin Symposium, Montpellier, France, APGI, 2004, 73-76.
Phosphinidene-capped cyclodextrins – A new generation of introverted ligands.
L. Poorters, D. Armspach, E. Engeldinger, D. Matt, Proceedings of the 12th International Cyclodextrin Symposium, Montpellier, France, APGI, 2004, 73-76.
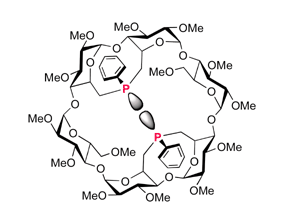
15.
Diastereospecific synthesis of phosphinidene-capped cyclodextrins leading to "introverted" ligands
E. Engeldinger, L. Poorters, D. Armspach, D. Matt, L. Toupet, Chem. Commun. 2004, 634-635.
Diastereospecific synthesis of phosphinidene-capped cyclodextrins leading to "introverted" ligands
E. Engeldinger, L. Poorters, D. Armspach, D. Matt, L. Toupet, Chem. Commun. 2004, 634-635.
14.
Conical cavitands as second coordination spheres and protecting environments. Towards metal-centred, intra-cavity reactions
D. Armspach, I. Bagatin, E. Engeldinger, J. Harrowfield, C. Lejeune, D. Matt, J. Iran. Chem. Soc. 2004, 1, 10-19.
Conical cavitands as second coordination spheres and protecting environments. Towards metal-centred, intra-cavity reactions
D. Armspach, I. Bagatin, E. Engeldinger, J. Harrowfield, C. Lejeune, D. Matt, J. Iran. Chem. Soc. 2004, 1, 10-19.
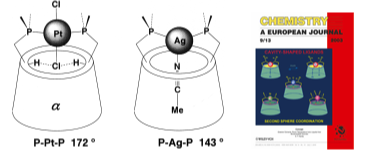
13.
Cyclodextrin phosphanes as first and second coordination sphere cavitands
E. Engeldinger, D. Armspach, D. Matt, P. G. Jones, Chem. Eur. J. 2003, 9, 3091-3105.
Cyclodextrin phosphanes as first and second coordination sphere cavitands
E. Engeldinger, D. Armspach, D. Matt, P. G. Jones, Chem. Eur. J. 2003, 9, 3091-3105.
12.
Cyclodextrin-encapsulated iron catalysts for the polymerization of ethylene
D. Armspach, D. Matt, F. Peruch, P. Lutz, Eur. J. Inorg. Chem. 2003, 805-809.
Cyclodextrin-encapsulated iron catalysts for the polymerization of ethylene
D. Armspach, D. Matt, F. Peruch, P. Lutz, Eur. J. Inorg. Chem. 2003, 805-809.
11.
Selective tetrafunctionalisation of α-cyclodextrin using the supertrityl protecting group - Synthesis of the first C2-symmetric tetraphosphane based on a cavitand (α-TEPHOS)
L. Poorters, D. Armspach, D. Matt, Eur. J. org. Chem. 2003, 1377-1381.
Selective tetrafunctionalisation of α-cyclodextrin using the supertrityl protecting group - Synthesis of the first C2-symmetric tetraphosphane based on a cavitand (α-TEPHOS)
L. Poorters, D. Armspach, D. Matt, Eur. J. org. Chem. 2003, 1377-1381.
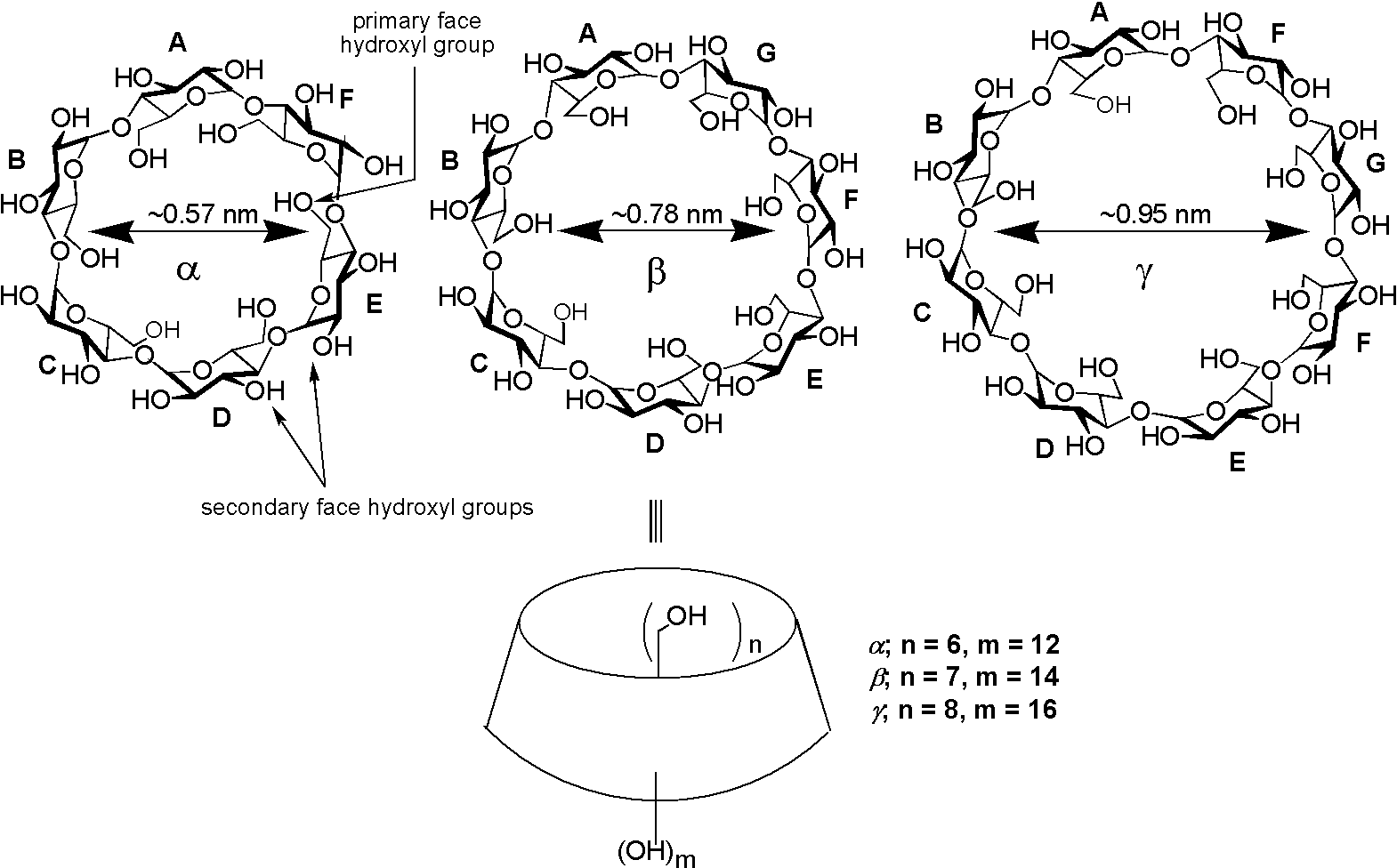
10.
Capped Cyclodextrins
E. Engeldinger, D. Armspach, D. Matt, Chem. Rev. 2003, 103, 4147-4174.
Capped Cyclodextrins
E. Engeldinger, D. Armspach, D. Matt, Chem. Rev. 2003, 103, 4147-4174.