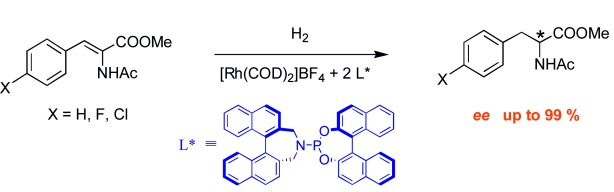
29.
BINOL-derived phosphoramidites in asymmetric hydrogenation: can the presence of a functionality in the amino group influence the catalytic outcome?
L. Eberhardt, D. Armspach, J. Harrowfield, D. Matt, Chem. Soc. Rev. 2008, 37, 839-864.
BINOL-derived phosphoramidites in asymmetric hydrogenation: can the presence of a functionality in the amino group influence the catalytic outcome?
L. Eberhardt, D. Armspach, J. Harrowfield, D. Matt, Chem. Soc. Rev. 2008, 37, 839-864.
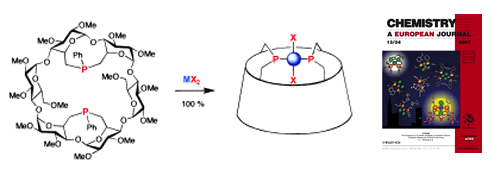
28.
Synthesis and properties of TRANSDIP, a rigid chelator built upon a cyclodextrin cavity. Is TRANSDIP an authentic trans-spanning ligand?
L. Poorters, D. Armspach, D. Matt, L. Toupet, S. Choua, P. Turek, Chem. Eur. J. 2007, 13, 9448-9461.
Synthesis and properties of TRANSDIP, a rigid chelator built upon a cyclodextrin cavity. Is TRANSDIP an authentic trans-spanning ligand?
L. Poorters, D. Armspach, D. Matt, L. Toupet, S. Choua, P. Turek, Chem. Eur. J. 2007, 13, 9448-9461.
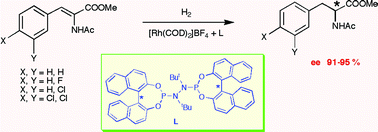
27.
Efficient asymmetric hydrogenation of olefins with hydrazine-derived diphosphoramidites
L. Eberhardt, D. Armspach, D. Matt, B. Oswald, L. Toupet, Org. Biomol. Chem. 2007, 5, 3340–3346.
Efficient asymmetric hydrogenation of olefins with hydrazine-derived diphosphoramidites
L. Eberhardt, D. Armspach, D. Matt, B. Oswald, L. Toupet, Org. Biomol. Chem. 2007, 5, 3340–3346.
26.
Chiral selectors for enantioresolution and quantitation of the antidepressant drug uoxetine in pharmaceutical formulations by 19F NMR spectroscopic method
M. Shamsipur, L. S. Dastjerdi, S. Haghgoo, D. Armspach, D. Matt, H. Y. Aboul-Enein Anal. Chim. Acta 2007, 601, 130-138.
Chiral selectors for enantioresolution and quantitation of the antidepressant drug uoxetine in pharmaceutical formulations by 19F NMR spectroscopic method
M. Shamsipur, L. S. Dastjerdi, S. Haghgoo, D. Armspach, D. Matt, H. Y. Aboul-Enein Anal. Chim. Acta 2007, 601, 130-138.
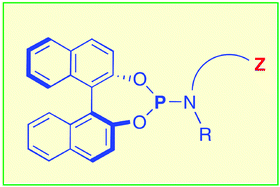
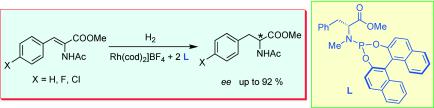
25.
Efficient, rhodium-catalyzed hydrogena tion of α-dehydroamino acid esters with chiral monodentate aminophosphanes bearing two binaphthyl groups
L. Eberhardt, D. Armspach, D. Matt, L. Toupet, B. Oswald, Eur. J. Org. Chem. 2007, 5395-5403.
Efficient, rhodium-catalyzed hydrogena tion of α-dehydroamino acid esters with chiral monodentate aminophosphanes bearing two binaphthyl groups
L. Eberhardt, D. Armspach, D. Matt, L. Toupet, B. Oswald, Eur. J. Org. Chem. 2007, 5395-5403.
24.
α-TEPHOS: A cyclodextrin-derived tetraphosphine for multiple metal binding
L. Poorters, D. Armspach, D. Matt, L. Toupet, Dalton Trans. 2007, 3195-3202.
α-TEPHOS: A cyclodextrin-derived tetraphosphine for multiple metal binding
L. Poorters, D. Armspach, D. Matt, L. Toupet, Dalton Trans. 2007, 3195-3202.
23. Synthesis of chiral, monodentate aminophosphine and phosphoramidite ligands derived from amino acid esters. Application in the Rh-catalysed asymmetric olefin hydrogenation
L. Eberhardt, D. Armspach, D. Matt, L. Toupet, B. Oswald, Eur. J. Inorg. Chem. 2007, 4153-4161.
L. Eberhardt, D. Armspach, D. Matt, L. Toupet, B. Oswald, Eur. J. Inorg. Chem. 2007, 4153-4161.
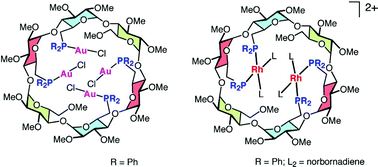
22.
A metallocavitand functioning as a container for anions. Formation of non-covalent, linear assemblies mediated by a cyclodextrin-entrapped NO3 anion
L. Poorters, D. Armspach, D. Matt, L. Toupet, Angew. Chem. Int. Ed. 2007, 46, 2663-2665.
A metallocavitand functioning as a container for anions. Formation of non-covalent, linear assemblies mediated by a cyclodextrin-entrapped NO3 anion
L. Poorters, D. Armspach, D. Matt, L. Toupet, Angew. Chem. Int. Ed. 2007, 46, 2663-2665.
21.
Cyclodextrin-based thiacavitands as building blocks for the construction of metallo-nanotubes
D. Armspach, L. Poorters, D. Matt, B. Benmerad, P. Jones, I. Dix, L. Toupet, J. Incl. Phenom. Macrocycl. Chem, 2007, 57, 243-250.
Cyclodextrin-based thiacavitands as building blocks for the construction of metallo-nanotubes
D. Armspach, L. Poorters, D. Matt, B. Benmerad, P. Jones, I. Dix, L. Toupet, J. Incl. Phenom. Macrocycl. Chem, 2007, 57, 243-250.
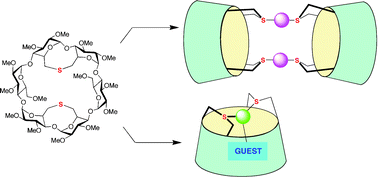
20.
Sulfur-capped cyclodextrins: a new class of cavitands with extroverted as well as introverted donor functionalities
B. Benmerad, P. Clair, D. Armspach, D Matt, F. Balegroune, L. Toupet, Chem. Commun. 2006, 2678-2680.
Sulfur-capped cyclodextrins: a new class of cavitands with extroverted as well as introverted donor functionalities
B. Benmerad, P. Clair, D. Armspach, D Matt, F. Balegroune, L. Toupet, Chem. Commun. 2006, 2678-2680.